What is a Cleanroom and Why Do You Need One?

Written by Macy Jenkins
What Is a Cleanroom?
A cleanroom is a regulated space that is designed to control contamination and aids in the removal of airborne particles. Cleanrooms are necessary for many industries where small particles can affect the manufacturing and quality of a product or assembly. Today, cleanrooms are usually found in industries such as pharmaceuticals, semiconductor, biotech, medical device, life sciences, optics, aerospace, automotive, and military facilities.
There are many sources of contamination that need to be facilitated including environmental control and personnel control. Cleanrooms can be filtered out by air handling units known as HEPA (High Efficiency Particulate Air) that removes 99.99% of particles. Some cleanrooms are maintained by temperature to reduce microbial growth like viruses, spores, fungi, and bacteria. Humidity control can also affect the static, and growth of microbes.
While a cleanroom might be sterile, people are not. In order to maintain cleanroom standards, personnel are required to garb up in non-particulating lab coats, booties, and hair nets. Annual testing is constructed based off the ISO 14644-1 standard that monitors the on-going formal testing measurements.
How Are Cleanroom Standards determined?
According to the National Science Foundation, “Facilities walls, floors, ceilings, paint, coatings, debris, and dust can all be a source of contamination. The equipment and supplies used as well as hair, skin oils, perfume, lint, fibers, and cosmetics play a factor in cleanliness.”

See Particulate Matter Basics via EPA
Cleanrooms are determined according to the degree of cleanliness the application requires. This also determines the contamination control maintained to meet the specifications. It is very important that these requirements are held to the classification standard from the beginning to the end of the production process or it may affect the quality and productivity of the assembly.
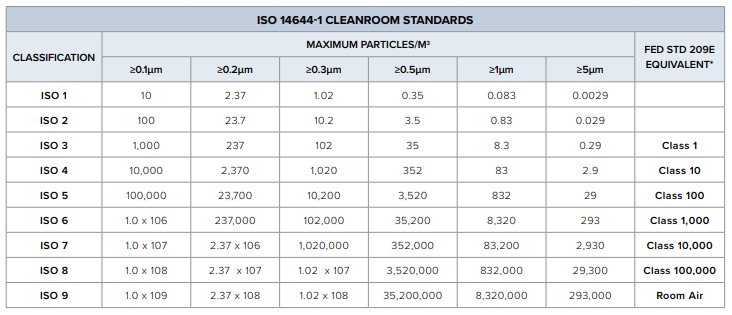
The number and size of the particles allowed in the room will determine the classification of air cleanliness. As the application in the cleanroom grows less critical, greater quantities of particles are allowed to be present without harm, making the numerical classification of the cleanroom higher. Cleanrooms are designed in various sizes and configurations and are rated based on the cleanroom industry ISO 14644-5:2004 standard and Federal Standard 209E. Once the classification of the cleanroom is determined, there are many requirements that should be followed to ensure the optimal cleanliness and control over the environment.
Benefits of a Cleanroom
What is the benefit of a contract manufacturer with cleanroom capabilities?
Cleanrooms are expensive to implement, maintain, and certify. Picking a contract manufacturer experienced with cleanroom facilities allows you to continue to maintain quality while reducing manufacturing costs.
There are actually three major benefits of manufacturing products in a cleanroom environment to control and reduce the number of airborne contaminants present.
Improves process consistency and part quality.
When particulates settle on surfaces of assembly devices such as molds, airborne contaminants can interfere with sensors, reduce material adhesion, and even optical clarity. Removing these contaminants from the air and reducing the amount of particulates settling on work surfaces inside the cleanroom can drastically decrease the yield loss rate of highly sensitive products. This in turn can enable the production process to produce higher quality parts more consistently at a lower cost, with less waste of parts.
Cleanroom packaging guarantees the cleanliness of the finished parts.
Because the air quality inside the cleanroom is free of particulates and microbes, unlike the air outside, the products and parts produced or packaged inside of the cleanroom will remain contaminant-free as long as manufacturers adhere to strict cleanroom operational procedure.
By preventing contamination of airborne microbes, cleanroom manufacturing processes reduce the risk to the ultimate end user. This allows the product to be immediately put into use without requiring sterilization after receiving the product, making it more cost-effective and quicker to deploy.
Meet FDA cleanliness and sterility requirements
The independent certification of a cleanroom, in addition to its materials, equipment, and associated procedures, are controls put in place to ensure clean products.
Integrating a cleanroom, its materials, and equipment into your process puts you in a strong position to prove that you can consistently produce clean, contaminant-free products.
OEMs can leverage contract manufacturers with cleanroom capabilities to protect the safety of the patients who rely on their products. This ensures consistent results for their production lines, helps to meet regulatory requirements, and their customers/end-users have confidence in the cleanliness of their products out in the market.
GMIs Class 10,000 Cleanroom
GMI Solutions invested in a class 10,000 positive pressure cleanroom. A positive pressure cleanroom is one of the many various cleanrooms out there. This system uses a HEPA system with high efficiency filters to remove particulates from entering the room as well as eliminates the settling of particulates in the room. With cleaned air flowing in and contaminates flowing out, positive air pressure minimizes the number and size of particle within the room. This system is great for applications we assemble like optics and gluing. GMI’s cleanroom is tested annually based off the ISO standard. The cleanroom is on a calibrated system where its testing will expire within a year of the issued date. When getting tested, the team takes multiple air samples within the space to get the number of particles per cubic foot. A class 10,000 cleanroom would have <10,000 particles per cubic foot and a minimum of 45 to 60 air changes per hour. GMI Solutions takes pride in never failing a test and keeping the cleanroom regulated and operational.
GMI Solutions invested in the class 10,000 positive air pressure cleanroom as a customer requirement by a global tier 1 medical device manufacturer who is known for their optics in the marketplace. The manufacturing of custom optical assemblies takes place in the cleanroom as well as a gluing and curing process. The cleanroom is a resourceful tool to GMI providing the ability to broaden our capabilities for new and existing opportunities.